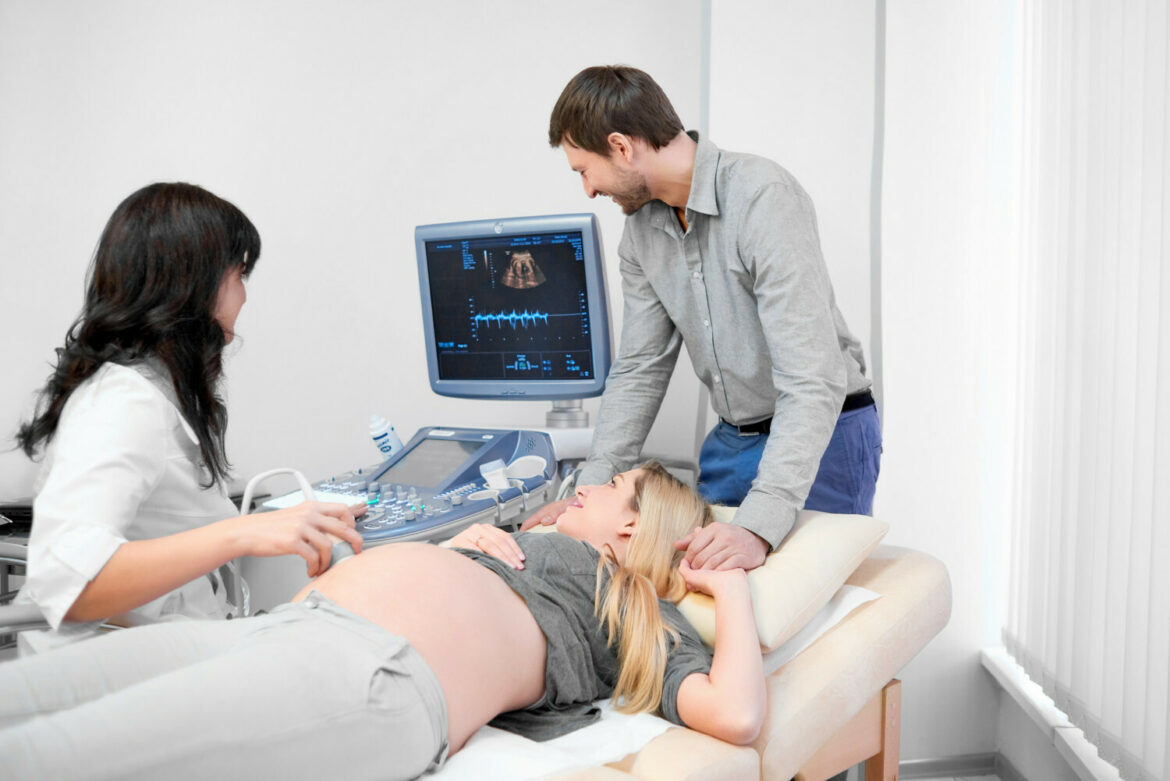
Researchers from the Gdansk University of Technology working in an international consortium will develop an innovative system based on artificial intelligence to assist pregnant patients. Among other things, the research team will create a ‘digital twin’ to support the monitoring of the patient’s condition at home and at the point of care.
The project will create a set of advanced tools, algorithms and services to collect and integrate data from the clinic (using point-of-care technology) and from individual measurements taken by the pregnant woman. Health history, vital signs, ultrasound and uterine artery flow measurements and a range of biomarkers will be available on the platform.
Through digital models, it will be possible to monitor and predict the risk of complications, such as pre-eclampsia and so-called gestational hypertension, even before symptoms appear. If necessary, the tools will help determine the optimal drug dose and treatment plan during pregnancy. The platform will be available for use by medical staff (doctors, nurses, midwives) and the patient and her relatives.
The international research consortium comprises Aarhus University and Aarhus University Hospital (Denmark), Gdansk University of Technology, Gameta Gdynia Centrum Zdrowia Sp. z o.o, and Zitec Com SRL (Romania). The project is supported by the National Centre for Research and Development.
The team from the Department of Biomedical Engineering at the Gdansk university is responsible for developing an artificial intelligence model for predicting the risk of diseases that can be caused by pregnancy. It will also be involved in developing methods for monitoring and measuring data from pregnant women. The Gdynia-based Gameta clinic, one of the largest centres for infertility treatment and prenatal testing in Poland, is involved in the preparation, implementation and evaluation of the results of the clinical trial.
Arkadiusz Słomczyński